Drug Naloxone is a lifesaving drug for patients suffering overdose of opioids it is available in united states on prescription, now naloxone will be available as otc drug as well, reducing the time for providing critical treatment for patients affected with opioid poisoning or overdose, naloxone reverses the effect of opioids quickly.
Narcan is the name of the approved drug by us fda, it contains naloxone 4 mg as nasal spray.
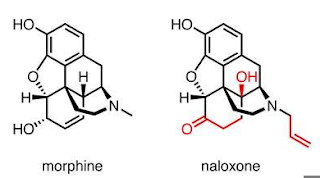
It is useful drug for patients who are addicted to opioid, naloxone reverses the effect of morphine and its derivate by competitively inhibiting the morphine receptors.
Drug naloxone act as an antagonist to morphine and its derivatives and opioids.
Antagonist drugs act on the same receptors as that of a particular molecule (here morphine) and blocks effect of the morphine, some antagonists do produce little similar effects as that of parent molecule that is targeted antagonistically.
Naloxone when given 1mg/kg of body weight is found safe and do not produce any effect on persons who have not exposed to morphine earlier or on a person who is not opioid dependent. While increase in dose up to 2mg/ kg may produce sedative effect, like sweating, nausea, inertia, dizziness, weakened cognition.
US FDA has approved the over the counter drug Narcan nasal spray naloxone 4 mg it is the first over the counter drug that is approved by US FDA which contains analog and antagonist for opioid.
When Naloxone containing nasal spray is given to a person who is opioid dependent, the drug naloxone prevents action of opioids like morphine due to which morphine or opioid action on patient’s body is stopped.
This is as good as withdrawal of the opioid, hence the person who is opioid dependent when started dosage of naloxone exhibits opioid withdrawal symptoms. Weakness increased blood pressure, increased heart rate (tachycardia) nervousness, restlessness or irritability.


No comments:
Post a Comment