How does it help to prevent contamination during aseptic filling of sterile dosage form?
Important points to learn in Restricted access barrier system design and development.
Main objective of the restricted access barrier system is to prevent contamination of sterile products during filling and also to maintain the level of cleanness of a clean room in which sterile product is filled aseptically.
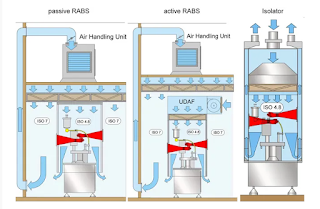
Restricted access barrier system is a system which actually forms an air tight barrier of hard SS frame walls, in which the sterile filling area with higher degree of cleanness which is almost sterile is enclosed. The walls or physical barriers physically prevent entry of humans or operators inside the area where the sterile product is filled.
Human or manual interference in the sterile filling activity a manmovement is one of the biggest critical reasons for generation of huge numbers of particles and bacterial and fungal contamination intern.
Human body and the growing harbors particles and bacterial contamination, the more the man movement greater is the risk of product failing in the sterility test. The Restricted access barriers provide solutions to the problem of human generated contamination.
The barriers surrounding the filling area are airtight, and gloves ports are provided on the sides for handling the product during filling without compromising the sterility.
Restricted access barrier system is provided with its own air filtration system with HEPA filter Unidirectional Air filtration unit. Which ensures the core filling area meets the Class A and the particle count is much lower than the class A. Entire filling line can be fitted inside the Restricted access barrier system. During aseptic filling operation RABS actually eliminates chances of contamination due to man almost 100%
Restricted access barrier system provided with following important facilities for smooth operations.
1) SS 316 walls are easily cleanable and can be decontaminated easily, smooth and rust free surfaces are desired in sterile filling areas.
2) Glove points through which manual intervention can be done by putting hands inside the gloves, and handling the product and machine during filling.
3) Ports for addition of externa material on the line like, media plates during, are samplers during the microbial evaluation of the aria.
4) Automated log, password protection and audit trials, in the machine that meets the requirement of 21 CFR part 211 for computer systems.
5) Facility to operate sometimes in an open door system.
6) Alarm systems for failure detection.
7) Clean in place and sterilize in place facilities for contact parts.
Systematically designed and qualified RABS is an important part in aseptic filling and sterile dosage form manufacturing.

No comments:
Post a Comment