Why is UV light used in water systems ?
Why pharmaceuticals use water systems to pass water through UV light?
Online dosing of water with sodium hypochlorite solution 1% during processing of purified water is just not sufficient, as during passing through ion exchange resin chlorine again is separated and deactivated, therefore when microorganisms initially treated with sodium hypochlorite solution
Water for pharmaceutical use is required to be very pure with respect to its purity, and microbial count of water is expected to maintained under stringent control, microorganism’s bacteria and virus can remain viable in water and up on getting in to suitable host or human body they can multiply and cause serious infection in human.
Therefore, water used in manufacturing of pharmaceutical dosage forms like liquid oral and solid oral dosage forms and topical dosage form is required to maintain microbial count under control. Total bacterial count allowed is 100 cfu but it is required to maintain within 20 cfu for a good water system. Total fungal count allowed is 10 but it should be NMT 2. Water should not contain any pathogenic microorganisms listed in pharmacopoeias.
Therefore, initially before passing the water from Mixed bed or cation and anion resin water is treated with sodium hypochlorite solution online dosing of 1 ppm solution.
Manufacturing unit is required to use the concentration as per the validated concentration that is effective in reducing the microbial contamination.
Chlorine dosing reduces the microbial count to acceptable levels, it depends on the contact time of chlorine with microorganism, to Agument antimicrobial activity, along with chlorine dosing UV light is used to decontaminate the water flowing in the pipes. Contact time of chlorine is less since water again enters ion exchange resin where chlorine is removed from water, the pretreated water with chlorine when pass through UV light tube, water is exposed to UV light. UV light causes damage to microbial DNA structure and further brings down the viable microbial count. UV light range is 180 nm to 280 nm.
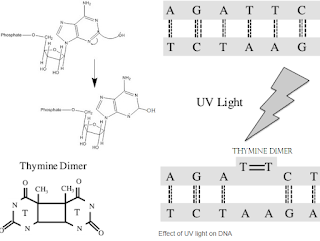 |
| Effect of Ultra Violet light on DNA base paring |
Mode of action of Ultra Violet light?
UV light photons are high energy photons, when it hit on bacterial or viral DNA or RNA the bases in the DNA or RNA get temporarily excited and electrons shifts occur because of which if the bases tend to pair with different bases for example pyrimidine and thymidine forms covalent bond with adjacent thymidine. as shown in the figure, which causes damage to DNA and bacterial or viral cells get killed.
The process is very effective in water which is already treated with 1 ppm sodium hypochlorite solution, it enhances the antibacterial action of UV light.
What is excision repair?
It is a process of cellular DNA or RNA repair, once a Bacterial or Viral DNA RNA is exposed to UV light and a damage occurs in DNA or RNA, the damage is reversed or repaired by a process called excision repair, enzyme DNA glycosylases breaks away the damaged bases of DNA fragments and allow for synthesis of new strands, the process is called excision repair.
Mode of action of Chlorine On bacteria and Virus?

No comments:
Post a Comment