European Union Guidelines doesn't make it mandatory to have separate man and material entry airlock while guidelines put emphasis on seprate man and material airlocks where ever possible. And where it's not possible it mentions that an adequate procedure and system should be in place to avoid cross contamination.
Airlocks are of three types
1.Casecade Airlock
2.Bubble Airlock
3. Sink Airlock
Depending on the requirement of the area Air locks are chosen.
An approach for pressure differential is given so that the air from outside area do not enter in to other core manufacturing area and contaminate air with different product dust particles.
Therefore always clean air should flow inside the inner core manufacturing area and inner product contaminated air should not enter in a passage which connects the other manufacturing area. To maintain this air flow direction pressure differential required to maintain.
Doors of the airlock should get closed in the direction of pressure higher to lower pressure. Otherwise they will remain open and add to product contamination.
Casecade Airlock there is higher pressure at outer corridor and then there is little lower inside the airlock, while inner core area has lower pressure than airlock. This system is used when one comman passage is connecting to many core manufacturing areas.
By doing this air pressure arrangement a casecade of pressure is generated. Such airlock are preferred in tablets dosage form where inner product, where the fine powder generation is more.
When the corridor is not common exactly reverse pressure differential is kept. Means inner core are has higher pressure than the Airlock and the passage has lower pressure than the Airlock.
Such casecade Airlock are used in tablets dosage form and topical preparation manufacturing.
Bubble Airlock: Here the Airlock has higher pressure than the outer corridor and inner core manufacturing area.The airlock too is supplied with HEPA filtered air and meets the required clean room Grade of cleanliness greater than inner core manufacturing area or almost equal to that.
Due to which when out side door of airlock is open the cleaner air gushes out in the corridor, and when inner core area door is opened the clean air from airlock goes in side the inner core manufacturing area. In both cases outer air is kept out, and inner air is kept inside. There is no chance of cross contamination.
Such types of airlocks are preferred in Injectible dosage form manufacturing. And where there is more chance of cross contamination.
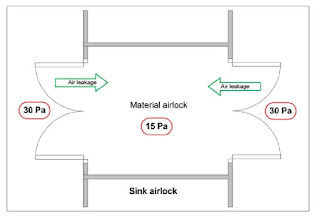
Sink Airlock:
Sink Airlock is reverse of bubble air lock, inside the sink air lock air pressure is lesser than outer corridor as well as inner core manufacturing area due to which air goes inside the airlock and taken out just like water sink basin.
It serves as a air drain point where contaminated air is taken out quickly to filtration and filter it and make it particle free.
Hope this information will provide you basic detail information about different types of airlocks used in pharmaceutical industry clean rooms.
Manometers are provided out of the clean rooms which monitors the pressure differential between outer corridor and inner core manufacturing area and airlock and outer as well as inner core manufacturing area.
10to 20 psi pressure differential is maintained between inner core area and outer corridor while 5 to 10 psi pressure differential is maintained between airlock and outer corridor.



No comments:
Post a Comment